Intervention plan to prevent and manage extravasation events for an Oncology Group in Brazil – UPDATE
Quality Improvement Challenge – INS 2024
Intervention plan to prevent and manage extravasation events for an Oncology Group in Brazil – UPDATE
First Author: Janyce Cassiolato Marti Sguassabia (Oncology Nurse)
Authors: Claudia Toledo de Andrade (Executive National Manager), Ana Flávia Almeida de Oliveira (IT Analist), Márcia Maria Domingues Vila Real (Risk Management Specialist), Mariana Laloni (Medical Director), Caroline Janzen (Nurse Educational Expert) and Taisi Feitosa (Educational Coordinator).
The authors would like to sincerely thank the unit’s leaderships: Luciana Cerqueira, Claynner Bessa, and Walter Maciel. To the registered nurses: Andressa Miranda, Fabiane Eleutério, Idira Soares, Nayara Nascimento and All nurses from Cancer Center in Belo Horizonte. To the Pharmaceutical Joyce Ferreira and the Patients and their Families. To the Dana-Farber team for their invaluable guidance during the development of this project.
Author contact: (+55) 71 999508282, jacassiolato@yahoo.com.br
Introduction:
The frequency of extravasations becomes relevant when adverse events associated with chemotherapy are investigated. However, from a statistical standpoint, the incidence of extravasation in the literature is low, reaching 1 – 7% of all target drugadministrations1,2,3. Nonetheless, consequences from extravasation can be devastating to patients who have a life-threatening illness and already experience significant emotional issues.
This Oncology group, which consists of 133 units throughout Brazil, recorded 99 extravasations during the period of 2020 to 2023. Considering that the extravasation of vesicant drugs is completely preventable and the significant impact that these events can have on patients’ quality of life, it is essential to implement actions to reduce the frequency of this complication.
Specific Aim:
Implement and standardize the extravasation management protocol and monitoring of indicators across the largest oncology group in Brazil (133 units), with the objective of reducing the number of extravasations of vesicant and irritant medications with vesicant potential to zero.
Methods:
From January 2020 to October 2023, 99 cases of extravasation of vesicant and irritant drugs with vesicant potential were reported in an Oncology Group with 133 units across Brazil.
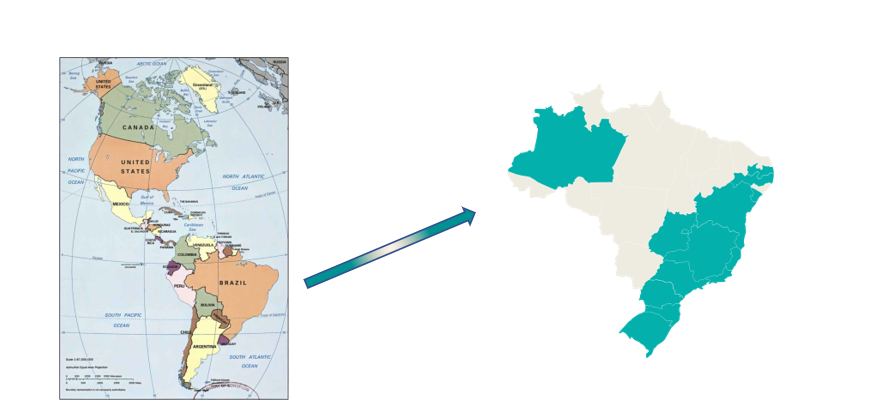
Figure 1: Map of Brazil demonstrating where the Oncology Group operates throughout Brazil ( in green).
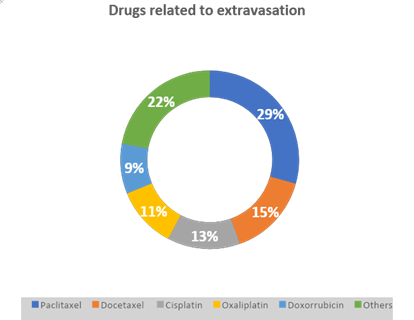
The most common medications involved were Paclitaxel 29.2%, Docetaxel 15.1%, Cisplatin 13.1%, Oxaliplatin 11.1%, Doxorubicin 9%, Irinotecan 8%, and other medications (22%), as you can see in the Graphic 1. No information has been collected regarding the impact on loss of affected limb function.
Graphic 1: Drugs related to extravasation
Quality Improvement Team:
The team was made up of the National Best Care Practices Manager, Executive Care Manager, Risk Management Specialist, Administrative Analyst and Care Unit Leaders.
Results
A group of nurses had a meeting to discuss the root cause exploration (Figure 2).
Figure 2: Root cause exploration
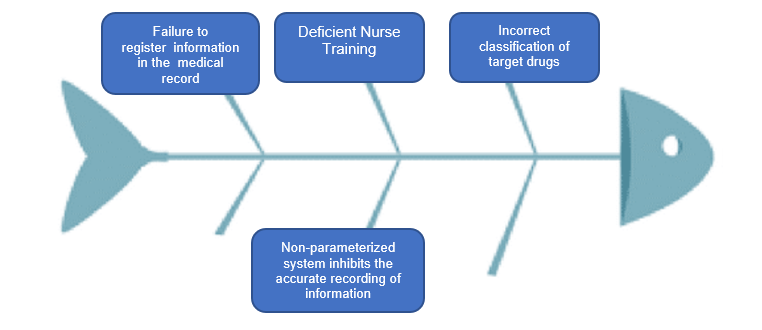
After the diagram was designed, the action plan was described in two groups: Educational and Process.
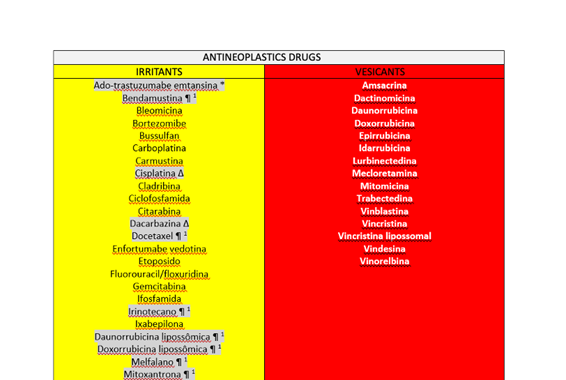
Educational Action 1: In July/2023, an extensive literature search was carried out to classify the vesicant and irritant medications with vesicant potential4,5 that are routinely administered by the Oncology Group as demonstrated in the Table 1.
Table 1: Classification of vesicant and irritant drugs
Educational Action 2: Subsequently, the description of the protocol was elaborated based on the International Guidelines of the Oncology Nursing Society (ONS), National Health Service (NHS) and Up to date as demonstrated in Figure 3.
Figure 3: Extravasation, Infiltration, and Phlebitis Management Protocol
This image is from the original document in Portuguese.
Educational Action 3: A nurse and a pharmacist were selected to record a Knowledge Track on this topic in video format. Technical validation forms for puncture and fully implantable catheters, as well as peripheral venous access and maintenance of local venous catheters were developed. Validation is scheduled to begin in December/2023, involving all nurses across the group’s units (Figure 4).
Figure 4: Image from the Knowledge Track about Extravasation (Audio and Video)

This image is from the original document in Portuguese. Image authorized by the professional
Educational Action 4: The patient record system was also improved to better evaluate the specific vesicant antineoplastics administered during the period, as well as to improve the indicator’s reliability and better record relevant information regarding these adverse events in the system.
Educational Action 5: In Brazil, about half of patients don’t have central venous catheters because of insurance coverage. Reassessment of the registration of medicines indicated for use with Infusion Pumps was a critical action to prevent the administration of targeted drugs via infusion pump using peripheral venous access. Together with the Commercial Division Patient Care Division, we were looking for an alternative to have better medication infusion control, and It started the use of a controlled infusion set used for vesicant infusions lasting >30 minutes (Figure 5).
Figure 5: Controlled infusion set

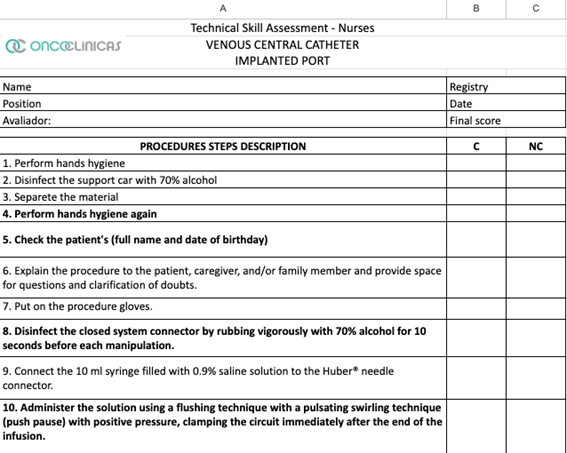
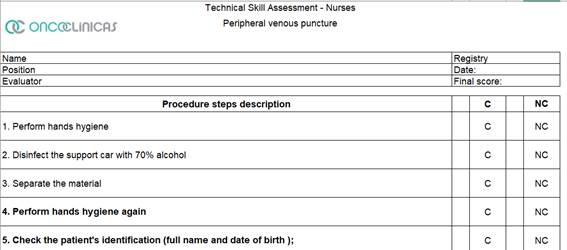
Educational Action 6: Professional assessment and Training “Key” Nursing Procedures such as Peripheral venous puncture, Port line puncture, and Central line permeabilization (Figure 6). The three-unit training began in November 2023 and ended in May 2024, with corporate staff present at four stages using the Technical Skill Assessment models demonstrated in Figure 7.
Figure 6: Pictures from the training

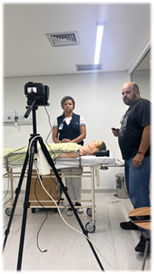
Image authorized by the professionals
Figure 7: Technical Skill Assessment – IMPLANTED PORT AND PIVC
It was necessary to retrain professionals who did not perform satisfactorily in the Technical Skills Assessment.
Process Action 1: Together with the Data teams, the Corporate Team reviewed the extravasation indicator adjustment according to international benchmarking. Defined as a goal Zero extravasation, for the future, but related to the extravasation historic in the oncology’s clinics, by 2024, the goal was to reduce the number of extravasation events by 20% per year.
Extravasation rate measures (indicator):
Calculation formula = (# Extravasations / # target drugs infused) x 100%
In May 2024, a questionnaire was applied to 53 oncology outpatient units to find out the number of peripheral and central venous access punctures (Port) performed during the period to calculate the representative sample of procedures for each unit. With the identification of the participating units, a general sample of 965 PIVC and 740 Port punctures was predicted and 758 peripheral intravenous catheters inserted (PIVC) + 630 Port punctures were achieved, data necessary for care leaders.
These punctures were divided among the 53 units where nursing assistants were chosen to observe the procedure and agreed to a 30-day deadline to carry out the audits. Each unit performed several punctures proportional to its monthly average. The clinical audit method with this group of nurse observers was chosen to expose opportunities for improvement more assertively. In July and August 2024, a form was used to conduct the clinical audit, describing the detailed technique and indicating whether or not each step complied (Figure 7). The observing nurses were instructed to detail each nonconformity observed. The objective of this audit was to observe whether there was standardization of best practices following international recommendations. The entire program was developed and implemented by two teams: the Care Management group and the Continuing Education group, both corporate, in partnership with the care leaders of the units. The observations were completed in August 2024. In phase 1, as a result, related to the forecast of punctures x puncture collection, 81% of the scheduled collections were obtained (758 + 965 punctures), distributed in 58% in the Southeast and South regions, 25% in the Central-West region and 16% in the Northeast region of Brazil. The steps identified with the highest number of nonconformities related to peripheral venous access puncture were lack of hand hygiene before putting on procedure gloves, failure to place the closed system connector immediately after performing the puncture, failure to disinfect the connector for 10 seconds and wash it with saline solution afterward to ensure that the access is patent. In the audit of the central access puncture, it was observed that the closed system connector was not used, that the swirling technique was not performed and that the dressing was inadequate. Some items were related to two procedures, such as disinfection of the tray and/or benches and using safety glasses as personal protective equipment.
Some justifications in the responses to the nonconforming items identified the need to expand the discussion over time, as well as form a materials standardization committee to standardize the closed system connectors in all groups and other materials relevant to safe practices.
DISCUSSION:
Standardizing hospital materials promotes patient safety and operational efficiency, ensuring that all units in the group have access to reliable, high-quality materials that are compatible with clinical and operational needs. The results obtained through the clinical audit showed the importance of reinforcing standards for technical procedures related to patient care. After the audits, all employees of the units participating in the proposal were instructed to undergo training on Improving Care for peripheral and central venous accesses that were available between August and October 2024. In addition to this intervention, a new audit was carried out in January 2025 with the observing nurses who used the same points on the form applied in the first audit to assess whether the intervention was successful after the training. The expected result for 2024 of a 20% reduction in overflows was not achieved, as 35 new cases of overflows were recorded, representing an increase of 17% compared to 2023.
Conclusion and next steps:
In the general context of actions proposed for 2024 and without yet evaluating the result of the new comparative audit (the results will come soon), it is essential to maintain continuous improvement actions, as a change in culture takes time to be effectively incorporated. The Standardization Committee was started in December 2024, and there was no time to define the structure and members to subsequently support the units in standardizing materials.
References:
1. Bahrami, Masoud1; Karimi, Tayebeh2,; Yadegarfar, Ghasem3; Norouzi, Ali4. Assessing the Quality of Existing Clinical Practice Guidelines for Chemotherapy Drug Extravasation by Appraisal of Guidelines for Research and Evaluation II. Iranian Journal of Nursing and Midwifery Research 24(6):p 410-416, Nov–Dec 2019.
2. Gorski LA, Hadaway L, Hagle ME, et al. Infusion Therapy Standards of Practice, 8th Edition. J Infus Nurs. 2021; 44 (1S Suppl 1):S1-S224. Available in: https://portaldeboaspraticas.iff.fiocruz.br/biblioteca/infusion-therapy-standards-of-practice-8th-edition/. Accessed on June 16, 2023.
3. Kim JT.,Park JY., Lee HJ., Cheon YJ. Guidelines for the management of extravasation. J Educ Eval Health Prof 2020; 17:21. Available in: https://www.ncbi.nlm.nih.gov/pmc/articles/PMC7431942/pdf/jeehp-17-21.pdf. Accessed on April 4, 2023.
4. NHS England. East Midlands Cancer Alliance. GUIDELINE FOR MANAGEMENT OF EXTRAVASATION. Jun, 2022. Available in:: https://www.eastmidlandscanceralliance.nhs.uk/images/Documents/SaCT/Guidelines_for_the_Management_of_Extravasation_2023.pdf . Accessed on April 05, 2023.
5. UPTODATE. Available in: https://www.uptodate.com/contents/search?search=extravasamento&sp=0&searchType=PLAIN_TEXT&source=USER_INPUT&searchControl=TOP_PULLDOWN&searchOffset=1&autoComplete=true&language=&max=0&index=0~10&autoCompleteTerm=extravasa&rawSentence. Accessed on May 17, 2023.







